Learning Outcomes
Students will be able to:
i. Define the second law of thermodynamics and explain its significance in understanding the direction of natural processes.
ii. Introduce the concept of entropy as a measure of disorder or randomness in a system.
iii. Explain the relationship between entropy and the second law of thermodynamics.
iv. Recognize the implications of the second law for various phenomena, such as heat transfer and energy transformations.
Introduction
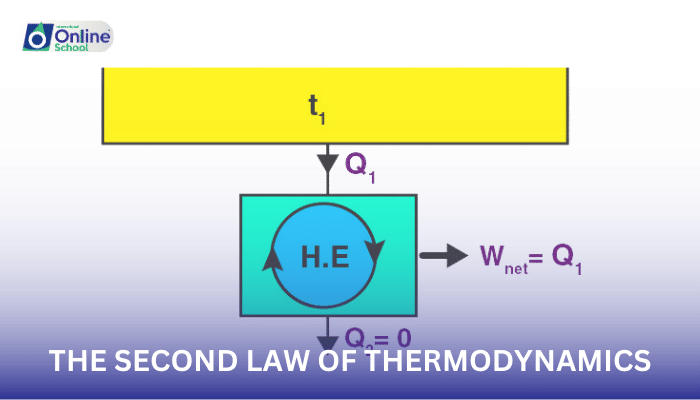
In the grand orchestra of nature, processes tend to move from order to disorder, driven by a fundamental principle known as the second law of thermodynamics. This law asserts that the entropy of an isolated system always increases over time. Entropy, a measure of the disorder or randomness of a system, provides a quantitative framework for understanding the direction of natural processes.
i. The Symphony of Order and Disorder: Unveiling Entropy
Imagine a room filled with scattered toys. The room is in a state of high disorder, with a high entropy value. If left undisturbed, the toys will likely remain scattered, maintaining the high entropy state. However, if the toys are neatly arranged on shelves, the room transitions to a state of lower disorder, with a lower entropy value.
This example illustrates the concept of entropy, a measure of the number of possible arrangements of a system's constituent particles. A system with a high entropy has a large number of possible arrangements, indicating a high degree of disorder. Conversely, a system with low entropy has a limited number of possible arrangements, indicating a high degree of order.
ii. The Second Law of Thermodynamics: A Symphony of Increasing Disorder
The second law of thermodynamics states that the entropy of an isolated system always increases over time. This means that natural processes tend to move towards a state of higher disorder, where the number of possible arrangements of the system's particles increases.
The increase in entropy is often associated with energy dissipation. For instance, when a hot object cools down, its internal energy decreases, and the energy is transferred to the surroundings as heat. This transfer of energy leads to an increase in the randomness and disorder of the surroundings, resulting in an overall increase in entropy.
iii. Implications of the Second Law: A Symphony of Limitations
The second law of thermodynamics has profound implications for various phenomena:
Heat Transfer: The second law governs the direction of heat transfer, explaining why heat flows spontaneously from hotter to colder objects.
Energy Transformations: The second law limits the efficiency of energy transformations, explaining why some energy is always lost as heat during energy conversions.
Direction of Processes: The second law determines the direction of natural processes, indicating that processes tend to move from order to disorder.
The second law of thermodynamics, a cornerstone of physics, provides a framework for understanding the direction of natural processes and the limitations of energy transformations. Its applications extend far beyond the realm of physics, shaping our understanding of various phenomena, from the cooling of a hot object to the behavior of complex systems. As we continue to explore the universe, the second law remains a guiding principle, illuminating the path to new discoveries and technological advancements.